CO₂ Utilization in the Chemical Industry: Turning Emissions into Valuable Products
Carbon dioxide (CO₂) has long been viewed solely as a pollutant—one of the main contributors to climate change. But thanks to rapid technological innovation, the chemical industry is now beginning to see CO₂ as something else entirely: a viable, sustainable raw material. From fuels and plastics to specialty chemicals, CO₂ is being transformed into high-value products through cutting-edge processes like catalysis, electrochemical reduction, and microbial synthesis.
This article explores:
✔ Breakthrough CO₂ conversion technologies
✔ Key products made from CO₂
✔ Industry leaders & future trends
1. Why CO₂ Utilization Matters
The Carbon Dilemma
Each year, the world emits over 40 billion tons of CO₂, mostly from energy production, industrial processes, and transportation. These emissions are driving global warming and threatening ecosystems, public health, and economic stability. Traditional carbon capture and storage (CCS) methods attempt to mitigate this impact by burying CO₂ underground—but they are often expensive, logistically complex, and lack a clear return on investment.
The Opportunity: Carbon Capture and Utilization (CCU)
Carbon Capture and Utilization (CCU) provides a more attractive alternative. Instead of merely storing CO₂, it turns emissions into value-added chemicals and fuels. CCU technologies can potentially displace up to 10–15% of fossil feedstock demand by 2030, according to McKinsey, while also supporting decarbonization efforts. For chemical manufacturers, CCU offers both a climate solution and a competitive edge.
2. Key CO₂ Conversion Technologies
1. Electrochemical CO₂ Reduction (eCO₂R)
This method uses electricity—ideally from renewable sources—alongside specialized catalysts to convert CO₂ into valuable chemicals such as formate, ethylene, or ethanol. These products serve as key building blocks in chemical manufacturing and energy sectors.
Advantages:
Enables on-demand, modular production
Utilizes excess renewable energy (e.g., solar or wind overcapacity)
Suitable for distributed, small-scale operations
Challenges:
Catalyst longevity and selectivity remain technical hurdles
Process efficiency must improve to scale economically
Industry Example:
Siemens Energy and Evonik collaborate on producing methanol from CO₂ and green hydrogen via electrolysis, proving the commercial viability of this method.
2. Thermocatalytic Hydrogenation
In this process, CO₂ reacts with green hydrogen over solid catalysts (like Cu/ZnO or Fe-based materials) to produce methanol, synthetic natural gas, or syngas. This method is particularly attractive because it integrates well with existing petrochemical infrastructure.
Advantages:
Easily scalable for large-volume applications
Leverages established reactor designs and distribution systems
Industry Example:
Carbon Recycling International (CRI) operates the largest CO₂-to-methanol plant in the world, located in Iceland, and demonstrates a closed-loop carbon strategy powered by renewable energy.
3. Biological CO₂ Fixation (Microbial & Algal Systems)
Here, engineered microorganisms and algae absorb CO₂ and convert it into compounds like ethanol, biodegradable plastics, and even proteins. These systems operate under ambient conditions and can process emissions directly from industrial flue gas.
Advantages:
Low energy input
Biocompatibility with waste gas streams
Scalable in bioreactors or photobioreactors
Industry Example:
LanzaTech uses proprietary bacterial strains to ferment steel mill off-gases, generating sustainable ethanol that is further used to produce jet fuel, cosmetics, and synthetic fibers.
4. Mineral Carbonation
Mineral carbonation captures CO₂ in a permanent, solid form by reacting it with calcium- or magnesium-rich materials to form stable carbonates. These carbonates can be used in construction materials like concrete blocks, effectively locking the carbon away for centuries.
Advantages:
Permanent CO₂ sequestration
Adds value in the construction and infrastructure sectors
Industry Example:
Carbicrete develops carbon-negative concrete blocks by curing cement-free formulations with captured CO₂, reducing both emissions and material costs.
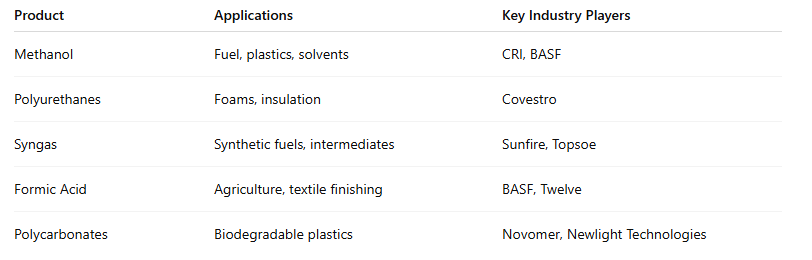
3. High-Value Products from CO₂
These CO₂-derived materials not only displace fossil-based inputs but also align with growing consumer and regulatory demand for sustainable, low-carbon products.
4. Challenges & Future Outlook
Barriers to Scale-Up
Despite the promise, several challenges must be addressed before widespread CO₂ utilization becomes mainstream:
High energy input for processes like electrolysis requires abundant, low-cost renewables.
Catalyst costs remain high, especially when using noble metals.
Policy gaps slow the adoption of CO₂-based materials due to inconsistent carbon pricing and limited incentives.
What’s Ahead
AI-Optimized Catalysts: Machine learning is accelerating the discovery of novel, affordable catalysts for CO₂ conversion, dramatically cutting R&D time.
Direct Air Capture (DAC): Companies like Climeworks are scaling DAC to provide high-purity CO₂ for chemical manufacturing, expanding feedstock availability.
Circular Carbon Economy: Aviation and shipping are beginning to use CO₂-derived fuels, completing the carbon loop and enabling net-zero operations.
Conclusion
CO₂ utilization is no longer a futuristic idea—it is becoming an integral part of the chemical industry's transition toward circularity and carbon neutrality. As the cost of renewables drops and technologies mature, converting emissions into revenue-generating products will redefine competitiveness in the sector.
Chemcopilot helps companies stay ahead by integrating CO₂ tracking into chemical process planning and design. With real-time calculation tools, Chemcopilot enables R&D teams and sustainability managers to quantify carbon footprints and identify low-emission alternatives—supporting smarter, greener innovation from lab to launch.