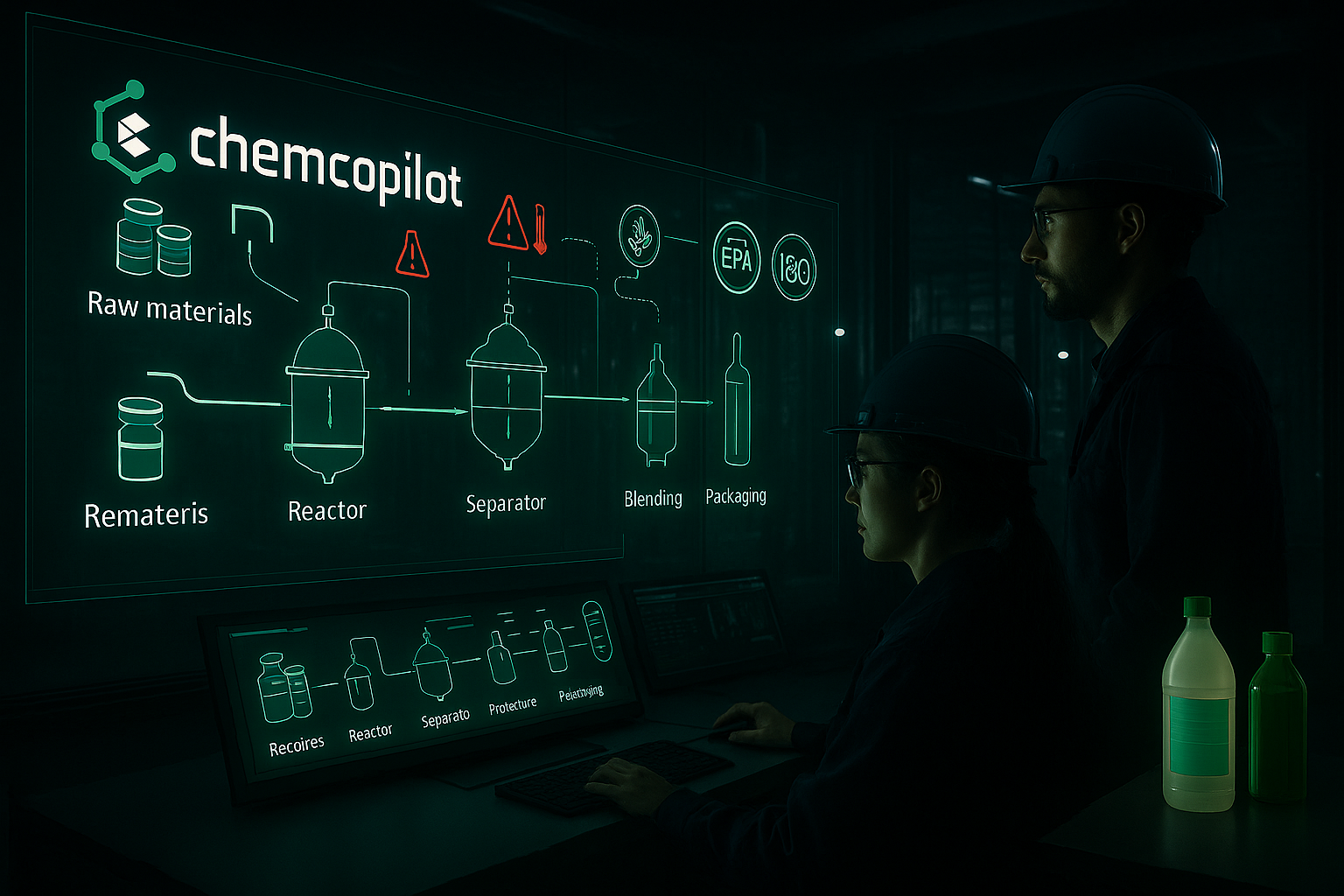
Why Chemical Manufacturers Need Stronger Batch Process Tracking for Quality and Traceability
Chemical manufacturing is the backbone of countless industries — from specialty coatings and plastics to agrochemicals, pharmaceuticals, food ingredients, and cosmetics. At the heart of this sector lies the multi-step batch process: a complex choreography of weighing, charging, reacting, separating, drying, blending, and packaging.
While much of today’s digital transformation conversation focuses on artificial intelligence, the immediate challenge for many chemical manufacturers is more fundamental: ensuring that every batch is traceable, compliant, and reproducible. A single deviation in a process parameter, an undocumented material substitution, or a missing batch record can cause off-spec products, safety risks, regulatory exposure, and costly recalls.
This article explores why batch process tracking and digital traceability are non-negotiable in chemical manufacturing, how companies can implement them effectively, and why this foundational step is essential before adding advanced AI layers.
The Complexity of Multi-Step Chemical Batch Processes
Chemical production, especially in batch mode, is inherently complex. Unlike continuous processes where materials flow steadily, batch production involves discrete, sequential steps. Each batch may take hours, days, or even weeks to complete — with hundreds of potential control points.
Critical stages include:
Raw Material Charging – verifying identity, lot number, and quality of raw inputs.
Reaction Control – maintaining precise temperature, pressure, pH, and mixing speeds.
Separation & Purification – distillation, filtration, crystallization, or drying to isolate desired compounds.
Blending & Formulation – ensuring homogeneity and consistency across multiple ingredients.
Packaging & Labeling – linking finished product back to its originating raw material lots.
At each stage, process parameters, operator actions, and material data must be logged. Failure to do so creates blind spots that compromise quality assurance and traceability.
For example, if a temperature excursion occurs during a reaction, manufacturers need the ability to instantly trace which raw material lot was affected, which operator was in charge, and which downstream batches might also be compromised.
The Risks of Poor Tracking in Chemical Manufacturing
When batch process tracking is inadequate, the consequences ripple across operations, compliance, and reputation.
1. Quality Failures
Without a complete digital trail, quality assurance teams struggle to identify the source of deviations. This often leads to entire batch rejections, even when only one parameter or lot may have been affected.
2. Regulatory Exposure
Regulators such as the EPA (United States), ECHA under REACH (Europe), and local environmental authorities worldwide demand proof of compliance and traceability. Missing or incomplete batch records can result in fines, product bans, or halted production lines.
3. Traceability Gaps
In industries such as food & beverage additives or agrochemicals, a recall without clear traceability is disastrous. Companies must sometimes recall entire product lines because they cannot pinpoint which lots are affected.
4. Operational Inefficiencies
When QA teams rely on paper logs or siloed spreadsheets, they spend more time verifying records than preventing issues. Investigations that should take hours often take weeks, delaying customer shipments and increasing costs.
5. Safety Risks
Incomplete tracking can mask hazardous conditions. For example, if a solvent was substituted with a different purity grade but not properly logged, it may create unsafe reaction conditions.
What Batch Tracking Systems Should Deliver
A modern batch management system must do more than digitize paper records. It should embed quality assurance and traceability into the fabric of chemical operations.
Core capabilities include:
Electronic Batch Records (eBRs): Replace manual paperwork with digital systems that capture every step in real time.
Lot Traceability: Link raw material lots to finished goods, ensuring recall precision.
Process Parameter Capture: Continuously log temperatures, pressures, concentrations, and times.
Deviation Alerts: Notify operators and QA teams immediately when parameters drift outside specifications.
Audit-Ready Documentation: Provide regulators with instant, tamper-proof access to batch histories (GMP, ISO, EPA, REACH compliance).
Integration with Quality Systems: Connect batch records with CAPA (Corrective and Preventive Actions), testing data, and laboratory systems.
End-to-End Visibility: Create a seamless digital thread from supplier input to customer delivery.
Real-World Scenarios in Chemical Manufacturing
Specialty Coatings
A batch of industrial paint fails viscosity testing. With digital batch records, the quality team can instantly trace back to the solvent lot number, mixing time, and temperature profile, identifying the root cause without halting unrelated production lines.
Agrochemicals
A regulatory inspection requires full traceability of an herbicide batch. A digital batch management system demonstrates compliance by linking raw material certificates, in-process tests, and final product specifications within seconds.
Food & Beverage Ingredients
A customer raises concerns about an additive’s taste deviation. Instead of manually combing through paper logs, the manufacturer uses digital records to trace the sugar lot, blending parameters, and filtration data that contributed to the batch.
Plastics & Polymers
A customer rejects a shipment due to inconsistent polymer properties. Digital traceability shows that a catalyst lot substitution caused minor variations, allowing targeted corrective actions instead of broad product recalls.
Regulatory and Industry Standards Driving Traceability
Chemical manufacturers operate under a patchwork of global regulations and industry standards. Strong batch process tracking helps companies align with these demands:
EPA (Environmental Protection Agency, USA): Ensures chemicals meet environmental and safety standards.
REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals, EU): Requires traceability of all substances manufactured or imported into the EU.
ISO 9001 (Quality Management): Emphasizes process control and traceability in manufacturing.
ISO 14001 (Environmental Management): Links environmental compliance with operational practices.
GMP (Good Manufacturing Practices): Essential for food, cosmetics, and pharmaceutical-related chemicals.
Responsible Care®: Industry initiative promoting safe and sustainable chemical manufacturing.
Strong tracking systems ensure that compliance audits are a matter of minutes, not weeks.
From Paper to Digital: The Evolution of Batch Tracking
For decades, chemical plants relied on paper-based batch records. While familiar, this system is:
Error-prone (handwriting, missing entries, illegible logs).
Slow to audit.
Nearly impossible to integrate with modern digital tools.
The shift toward electronic batch records (eBRs) and manufacturing execution systems (MES) marks a turning point. With these systems:
Operators record data in real time via tablets or terminals.
Sensors and IoT devices automatically capture process parameters.
Data flows directly into quality and regulatory reporting systems.
The result: accuracy, speed, and trustworthiness in every batch.
The Role of Chemcopilot in Batch Process Tracking
Chemcopilot’s mission is to support chemical manufacturers in building a digital backbone for traceability and compliance. By integrating regulatory parameters instantly into workflows, Chemcopilot enables:
Seamless batch records that combine operator inputs, sensor data, and material tracking.
Automated compliance checks for EPA, REACH, and other regulations.
Deviation management with AI-supported analysis, once the foundational data is secured.
Sustainability integration, linking batch processes to CO₂ calculation and lifecycle assessment, helping companies track environmental impact alongside quality.
Building the Foundation Before AI
Many manufacturers ask: “Can AI optimize my batch processes?” The answer is yes — but not before building strong digital fundamentals.
AI thrives on clean, structured, and complete data. If batch records are fragmented across paper files, spreadsheets, and disconnected systems, AI will only amplify noise.
The roadmap should be:
Digitalize batch process tracking.
Establish end-to-end traceability.
Ensure compliance and audit-readiness.
Layer AI on top for optimization, prediction, and automation.
This progression ensures that AI becomes a value-adding tool, not a band-aid for broken records.
Future Outlook: Traceability as a Competitive Advantage
As markets become more regulated and customers demand greater transparency, traceability will no longer be optional. Companies that invest in strong batch tracking will benefit from:
Faster product releases thanks to efficient QA.
Lower compliance risks with audit-ready records.
Improved customer trust through proven traceability.
Enhanced sustainability metrics, tying production data to carbon footprint and lifecycle assessments.
In a competitive chemical industry, these factors are not just about compliance — they are about differentiation and long-term growth.
Conclusion
For chemical manufacturers, the first digital transformation priority is not AI, but robust batch process tracking. By ensuring that every step, material, and parameter is recorded and traceable, companies safeguard quality, compliance, and safety.
With this foundation in place, advanced analytics and AI can later be layered to optimize production, predict deviations, and accelerate innovation. But without reliable digital batch tracking, these advanced tools will lack the structured data they need.
The message is clear: traceability first, AI second. Companies that embrace this sequencing will not only protect their operations today but also position themselves to lead in the chemical industry of tomorrow.